Determination of NaCl with very low content (Spectrophotometry)
According to GB/T11213.2-2007, NaCl content can be measured between 0.0002%-0.02%
Principle
The chloride ion (Cl) in the sample all replaced the thiocyanate group (SCN -) in mercury thiocyanate, and the replaced thiocyanate group (SCNT) reacted with ferric nitrate to produce iron thiocyanate, which showed red color. The colored solution was photometric determined at the wavelength of 450 nm. The reaction formula is as follows:
2NaCl+Hg(SCN)2-→HgCl+-2NaSCN
3NaSCN+Fe(NO3)3——→3NaNO3+Fe(SCN)3
Reagents and materials
The reagents and water used in this method are all analytical pure reagents and tertiary water specified in GB/T 6682 or water of corresponding purity. Standard solutions, preparations and products required in the test shall be prepared according to GB/T 603 except as specified in this part. Preparation, storage, sampling and determination of reagents should be carried out in an environment free of chlorine and hydrogen chloride.
Nitric acid。
Ferric nitrate[Fe(NO3)3- 9H2O].
Hydrogen peroxide。
Ferric nitrate solution :8 g/L(in Fe).
Two preparation methods of ferric nitrate solution can be selected.
Method 1: In a 500 mL conical bottle, add about 4.0g pure iron (purity > 99.5%), accurate to 0.01g, add 80 mL of water, and then carefully add 80 mL of nitric acid, slowly heat the solution to boiling in the fume cabinet, after the reaction is completed, and all nitrous acid gas is removed, add a few drops of hydrogen peroxide, so that the solution is decolorized, continue to boil for 2 min, stop heating, and move the solution into 500 after cooling In mL volumetric bottle, dilute to scale with water and shake well.
Method 2: In a 500 mL conical bottle, add about 29.0 g of iron nitrate [Fe(NO3)3·9H2O], accurate to 0.01 g, add 60 mL of water, and carefully add 60 mL nitric acid, slowly heat the solution to boiling in the fume hood, after the reaction is completed and all nitrous acid gas is removed, add a few drops of hydrogen peroxide to decolorize the solution, continue to boil for 2 min, stop heating, after cooling, transfer all the solution into a 500 mL volumetric bottle, dilute it with water to the scale, and shake well.
Mercury thiocyanate solution:0.5 g/L。
Weigh 0.1g mercury thiocyanate [Hg(SCN)2], weigh to 0.001 g, place in a 250 mL beaker, add 30 mL anhydrous ethanol, stir continuously, and add 150 mL warm water to dissolve it. Then, filter the solution into a 200 mL volumetric bottle, dilute it with water to the scale, and shake well.
Sodium chloride standard solution:10mg/L。
Phenolphthalein indicator solution:10 g/L。
Instruments and equipment
MacylabUV-1200 spectrophotometer
Analysis procedure
Drawing of standard curves
Preparation of standard colorimetric solution
The standard solution of 0.0 mL,2.0 mL,4.0 mL,6.0 mL,8.0 mL,10.0 mL,12.0 mL and 15.0 mL sodium chloride was absorbed successively and placed into 50 mL volumetric bottles, and then 5 mL nitric acid,5 mL ferric nitrate solution and 20 mL were added to each volumetric bottle successively mL mercury thiocyanate solution, dilute to scale with water, shake well, leave for 30 min to develop color.
Determination of absorbance of standard colorimetric solutions
With a spectrophotometer, the zero point of the spectrophotometer was adjusted with water at the wavelength of 450 nm, and the absorbance was determined by using a 4 cm or 5 cm absorber.
Drawing of standard curves
The mass (mg) of sodium chloride in 50 mL standard colorimetric solution is taken as the horizontal coordinate, its corresponding absorbance is taken as the vertical coordinate, and the absorbance of the blank solution is deducted to draw the standard curve.
Preparation of sample solution
Weigh a solid or liquid laboratory sample equivalent to 20g sodium hydroxide, weigh to 0.01g, dissolve water in a 200 mL volumetric bottle, and dilute to scale. Shake well.
When the mass of sodium chloride in 50 mL standard colorimetric solution is greater than 0.15 mg, absorb the sample solution, dilute the appropriate ratio, and determine the operation. The calculation result is based on the dilution ratio of the sample solution, and formula (1) is multiplied by the corresponding dilution ratio.
Blank test
The blank test is carried out at the same time as the sample determination, and the test procedure and dose are the same as that used in the sample determination, except that the sample solution and nitric acid used in the neutralization of the sample are not added.
Measure
Absorb 10.0 mL sample solution (6.2), place it in a 50 mL volumetric bottle, add 1~2 drops of phenolphthalide as indicator, shake it in water while slowly adding nitric acid to neutralize it, cool it to room temperature, add 5.0 mL nitric acid,5.0 mL ferric nitrate solution and 20.0 mL mercury thiocyanate solution, dilute it with water to the scale, shake well, and stand for 30 min color rendering.
Result calculation
The mass fraction w of sodium chloride, expressed as %, is calculated according to formula (1) :
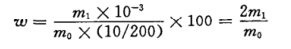
Formula:
m0 -- the value of the sample mass in grams (g),
m - The value of the mass of sodium chloride corresponding to the absorbance of the tested sample obtained from the standard curve, in milligrams (mg).



